We are committed to providing quality and safe cosmetic products to all our consumers, medical professionals (physicians, dermatologists) and beauty professionals (hairdressers, beauticians).

To ensure our products are safe, we go through an in-depth 4-step evaluation process that begins at the earliest stages of product conception and continues once the products have been released to market:
-
In-depth knowledge of raw materials that compose our cosmetic products
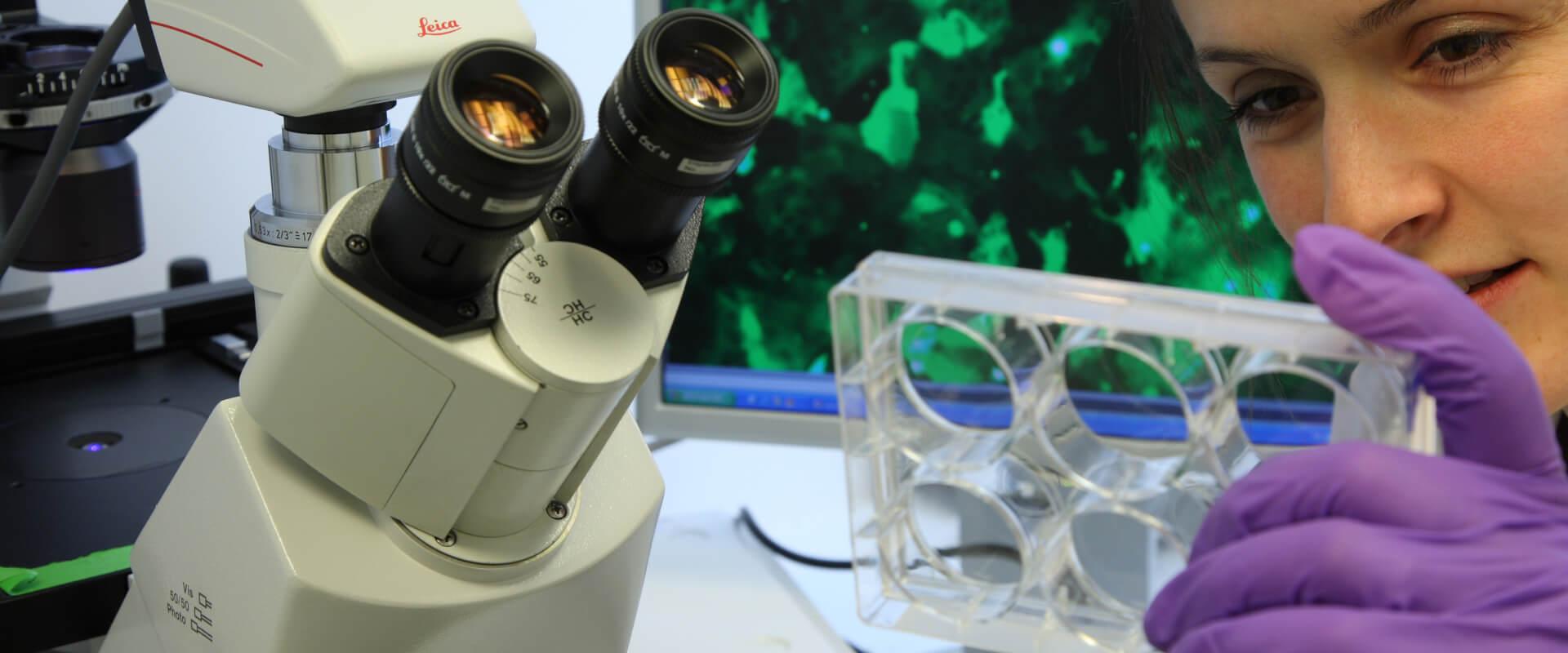
The safety evaluation of a product always starts with a deep knowledge of the raw materials we use. Having a thorough understanding of their quality, origin, or allergenic potential, among other facts, is possible thanks to the available best-in-class scientific data as well as rigorous tests carried out by internal experts. Each ingredient has its own "ID card".
-
Assessment of the use of raw materials in our products
This step is essential in product safety evaluation. It consists in evaluating the potential hazard of each raw material by a set of key parameters, linked to the exposure of the human body to these raw materials, which will enable its safe use in the finished product.
These weighing factors incorporate first and foremost the product category.
Thus, the exposure will be different between rinse-off products (like shampoo or body wash) and leave-on products after use (such as skincare products) or between products applied to the full body (like body milk) and those applied to a smaller area (like mascara). The weighing factors also include the frequency of product use and certain consumer characteristics which impose certain restrictions (products destined for children or for sensitive skin, for example).
Following this stage, a maximum safe concentration of each raw material in the product is determined. In order to ensure a wide safety margin for the consumer, the concentrations in the finished product are always at least 100 times lower than the no-effect dose. -
Confirmation of product tolerance via a wide range of tests
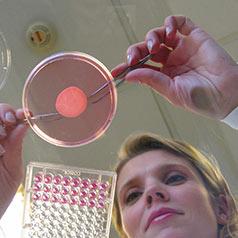
Once we have verified that each raw material can be used under doses that present no risk to the consumer, we then go about confirming the safety of the finished product under normal or foreseeable conditions of use (while taking into account possible misuses) in order to detect even the smallest undesirable effect for the consumer.
To do so, we first conduct an analysis comparing our new products with our extensive existing clinical database.
We also subject them to complementary in vitro safety tests and clinical tests conducted on healthy volunteers constituting particular consumer groups (people with sensitive skin for example).
The studies are conducted in specialized third party laboratories and research centers and are undertaken within a strict methodological and ethical framework. When this last stage has been successfully completed, the individual in charge of the safety assessment is in a position to finalize and sign the product safety report, which will then be included in the regulatory dossier on the product.
In addition to this thorough process, a fourth step occurs once the finished product has been released to market and is commercialized. -
Continuous monitoring of our cosmetic products after their release to market
We carry out a strict monitoring of the security of our products as soon as they’re released to market, and this is the case everywhere in the world.
This is possible thanks to our international cosmetic safety monitoring network which collects and analyzes the possible undesirable effects after use of our products, reported by consumers or healthcare professionals.
Indeed, as an example, it can happen that a person develops an allergy to an ingredient that is safe for the rest of the population.
In case of unwanted effects, even very benign or reported by a small number of consumers, we can decide to adjust product composition, in order to ensure our consumers are confident in using our products.